Abstract
Evolutionary adaptations to high altitude in Tibetans, Ethiopians, and Andean populations of South America have shown that Tibetans and Ethiopians have normal hemoglobin %, while most of Aymara and Quechua of the Andean highlands are polycythemic. Whole genome sequencing (WGS) in Quechua identified enriched SENP1 and ANP32D genes correlating with polycythemia (Zhou et al, Am J Hum Genet. 2013 Sep 5; 93(3): 452-462) but these genes were neither enriched nor segregated with polycythemia in Aymara. Instead, we identified that genes enriched in Aymara are related to regulation of cardiovascular development in high-altitude adapted Andeans, BRINP3, NOS2, and TBX5 (Crawford et al, Am J Hum Genet. 2017 Nov 2;101(5):752-767).
To further search for Aymara propensity to polycythemia, we analyzed transcriptomes from Aymara and Europeans living in La Paz, Bolivia (3,639-4,150m) from limited amount of peripheral blood reticulocytes, platelets and granulocytes, but only granulocyte RNA was adequate for unbiased whole transcriptome analyses. In Aymaras, 2,585 genes were upregulated and 365 genes were downregulated (Adjp<0.05, fold difference <-2.0, and >2.0). Many of these modulated genes are involved in inflammatory pathways including B-cell activation (FDR=0.005) and NF-κB signaling pathway (FDR=0.011). We then analyzed differential exon usage in the transcriptome and identified 2,475 genes with alternative splicing events, comprising 1,568 exon skipping, 485 intron retention, 175 alternative 3' splice sites, 144 alternative 5' splice sites, and 902 mutually exclusive exons. These alternative spliced genes were also overrepresented in inflammatory pathways (TNF receptor, IL-1 and IL-23 mediated signaling, and NF-κB signaling). Notably we detected the previously unreported NFKB1 alternate transcripts skipping exon 4 or 5, which lead to the out-of-framed NFKB1 mRNA, generating the truncated nonfunctional NF-κB protein (Figure). Inflammation is a potent suppressor of erythropoiesis and the NF-κB is transcriptional regulator of plethora of inflammatory genes. Further, NF-κB also interacts with erythropoiesis-regulators, hypoxia-inducible factors (HIFs).
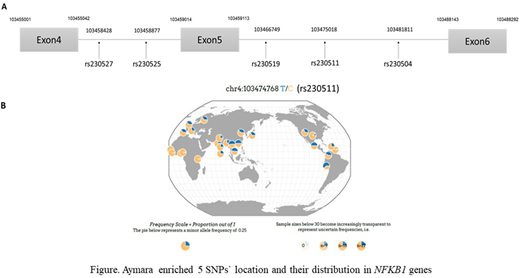
By the integrative analysis of the Aymara transcriptome and WGS, we identified 46 NFKB1 splicing quantitative trait loci (sQTLs). Among these 46 sQTLs, five single nucleotide polymorphisms (SNP) were in high linkage disequilibrium, and two (rs230511 and rs230504) were more enriched in Aymara (allele frequency: 0.878) (Figure) and within a genomic region where Andeans are genetically differentiated from lowland Native Americans (peak FST = 0.37, peak PBSn1 = 0.31). These sQTLs rs230511 and rs230504 were corelated with two functionally important exon skipping (exon 4 and 5) in NFKB1 as described above. Furthermore, these two SNPs were correlated with higher hemoglobin levels and lower leukocytes; the wild-type NFKB1 transcript inversely correlated with hemoglobin%.
We report Aymara have differentially expressed and alternatively spliced transcripts of genes modulating inflammation, particularly NFKB1. This Aymara enriched NFKB1 haplotype variant stands out as a major cause of Aymara adaptation to high altitude, as this truncated nonfunctional NF-κB variant peptide correlates with higher hemoglobin, lower leukocytes and suppresses inflammation. These data indicate that NFKB1 SNPs enriched in Aymara are associated with alternative spliced NFKB1 transcripts which contribute to polycythemia in Aymara. Further evaluation of NF-κB and HIFs' transcriptional activity and their correlation with inflammatory makers, hepcidin and erythroferrone in Aymara and Europeans living at the same high altitude is under way.
JS and SH contributed equally to this work. YL and JTP act as equivalent co-senior authors.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal